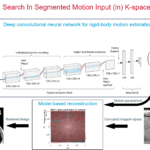
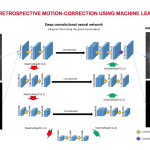
SISMIK: Search In Segmented Motion Input (in) K-space
Description: To tackle the problem of motion artifacts in brain MRI, we propose a deep-learning based approach for rigid-body motion estimation directly in k-space coupled with a model-based reconstruction method to avoid the risk of deep hallucinations. By simulating realistic motion artifacts in motion-free brain MRI scans, we have enabled the generation of large datasets, which is a requirement for training data-hungry deep learning models. SISMIK has the potential to be adapted to a wide range of MRI pulse sequences and k-space trajectories. We hope that the research and clinical communities will benefit from our method..
Investigators: Oscar Dabrowski (UNIGE), Sébastien Courvoisier (HUG), Jean-Luc Falcone (UNIGE), Bastien Chopard (UNIGE), Francois Lazeyras (HUG), Frédéric Grouiller (HUG)